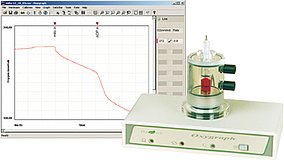
Clark electrode
Principle:
The Clark type electrode uses a silver/platnium-electrode immersed in an electolyte solution. An oxygen-permeable membrane, which encloses the electrode, enables the measurement of oxygen exchange between the test sample and the electrolyte solution. As the potential increases, oxygen is reduced to hydrogen peroxide when electrons are donated to oxygen. The current flow is stoichiometrically related to the oxygen consumed at the electrode.
Specifications:
- Peltier-Oxygen unit with integrated thermo-electrical temperature control
- Compact design with integrated electronic devices and integrated magnetic stirrer
- Oxygen-electrode system with online monitoring
Applications:
- Measurement of oxygen rates in solutions
- Blood oxygen levels
- Biochemical oxygen consumption (respiration, photosynthesis)
- Oxidation stabiltiy of liquids